EU backs curbs chinese medical device firms bidding public tenders, setting a precedent that could reshape global medical device procurement. This action highlights the EU’s commitment to safeguarding its healthcare sector and potentially influencing international trade practices. The move reflects a complex interplay of regulatory differences, economic considerations, and geopolitical tensions. The EU’s rationale for these curbs will be examined, along with potential impacts on both the EU and Chinese markets.
EU regulations regarding medical devices have evolved significantly over time, adapting to changing global trade dynamics and safety concerns. This evolution has led to a nuanced approach to international procurement, aiming to balance openness with rigorous standards. The EU’s existing policies governing public tenders for medical devices will be analyzed, alongside a comparative look at similar regulations in other major global economies.
Background on EU Regulations
The EU’s approach to medical device regulations has evolved significantly over time, driven by a commitment to patient safety and innovation. Early regulations focused primarily on ensuring basic quality and safety standards. This framework has progressively become more sophisticated, reflecting advancements in technology and the growing global market for medical devices. The EU’s regulatory landscape is now crucial for both domestic manufacturers and international competitors seeking to access the lucrative European market.The EU’s regulations, far from being static, are continuously updated and adapted to address new challenges and opportunities.
This adaptability is essential to maintain a balance between protecting public health and promoting a thriving medical device industry, both within the EU and internationally. The regulatory path for medical devices is intricately intertwined with global trade and procurement, shaping how companies from all corners of the world approach the European market.
Historical Overview of EU Medical Device Regulations
The EU’s journey in regulating medical devices began with a series of directives, each addressing specific aspects of the industry. These directives were gradually replaced by the current EU Medical Device Regulation (MDR) in 2017. The MDR represents a significant shift, moving from a directive-based approach to a more harmonized and comprehensive regulatory framework. This evolution reflects the EU’s commitment to a unified approach to medical device safety across its member states.
The earlier directives, though effective in their time, lacked the flexibility and adaptability needed to keep pace with technological advancements. The MDR, with its stringent requirements and clear responsibilities, aims to address these shortcomings.
Evolution of Regulations Concerning International Trade and Procurement
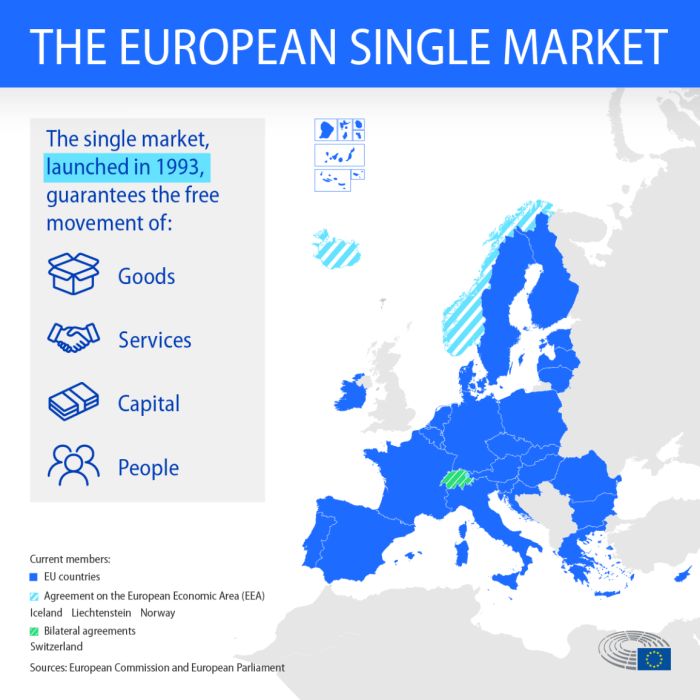
Initially, the EU’s approach to medical device regulations had a more fragmented effect on international trade. Diverse standards across member states created complexities for manufacturers seeking to operate in the EU market. The adoption of the MDR aimed to create a single, unified regulatory environment, reducing these complexities. This harmonization significantly reduced barriers to entry for foreign manufacturers. The EU now has a transparent and predictable regulatory landscape for international players seeking to penetrate the European market.
This unified approach facilitates a level playing field for all participants, regardless of their geographical origin.
Rationale Behind the EU’s Approach to Medical Device Procurement
The EU’s approach to medical device procurement is driven by a blend of factors. Patient safety is paramount, and the EU seeks to ensure that medical devices used within its borders meet stringent safety standards. Another key driver is fostering innovation. The EU recognizes the importance of a supportive regulatory environment for medical device companies, encouraging research and development.
The EU wants to promote innovation by encouraging research and development in the medical device sector. Furthermore, EU procurement policies aim to promote competition and transparency, leading to more efficient and cost-effective healthcare systems.
The EU’s move to restrict Chinese medical device firms from bidding on public tenders is a significant step, potentially impacting global healthcare access. While this highlights concerns about foreign influence, it’s interesting to note that a virtual chronic care provider, Omada Health , recently raised $150 million in an IPO. This suggests a growing market for digital health solutions, which could potentially offer alternatives to traditional methods, and ultimately, influence the broader landscape of medical device procurement in the future.
Existing EU Policies Governing Public Tenders for Medical Devices
EU public procurement policies are designed to ensure fairness and transparency in the selection of medical devices for public use. These policies mandate that procurement processes are open, competitive, and based on clear criteria. Specifically, EU rules require that tenders are advertised widely, providing ample opportunity for all qualified bidders to participate. This approach aims to maximize competition, leading to better value for money for public healthcare systems.
Comparison of EU Medical Device Regulations with Other Major Economies
| Characteristic | EU (MDR) | USA (FDA) | Japan (PMDA) | China (CFDA) |
|---|---|---|---|---|
| Regulatory Approach | Harmonized, unified regulation | Agency-based, performance-oriented | Combination of guidelines and regulations | Combination of guidelines and regulations, increasing emphasis on harmonization |
| Standardization | Strong emphasis on international standards | Focus on safety and effectiveness | Extensive use of national standards | Emphasis on international standards, with national standards |
| Market Access | Harmonized market access, but complex procedures | Relatively straightforward market access but potentially varied requirements by product type | Complex market access, but increasingly aligned with international standards | Complex market access, with growing emphasis on international standards |
| Enforcement | Strong enforcement mechanisms | Strong enforcement mechanisms | Strong enforcement mechanisms | Enforcement mechanisms are becoming stronger, but challenges remain |
The table above provides a basic overview of the regulatory environments for medical devices in major economies. Each jurisdiction has its own specific strengths and weaknesses, which companies must carefully consider when seeking to enter or expand in a given market. The increasing emphasis on harmonization and international standards reflects a global movement towards more standardized and interconnected medical device markets.
Overview of Chinese Medical Device Industry
The Chinese medical device industry has undergone remarkable growth in recent decades, fueled by a burgeoning middle class, increasing healthcare awareness, and significant government support. This expansion has positioned China as a major player in the global medical device market, presenting both opportunities and challenges for international competitors. Understanding this dynamic landscape is crucial for businesses navigating the complexities of global trade in medical technology.The Chinese medical device industry’s rapid development reflects a combination of factors, including government policies promoting domestic innovation, substantial investments in research and development, and a burgeoning healthcare infrastructure.
This growth is not without its complexities, however. Challenges such as maintaining quality control, harmonizing regulatory frameworks with international standards, and addressing intellectual property concerns are crucial to navigating this evolving market.
Growth and Development of the Chinese Medical Device Industry
The Chinese medical device industry has experienced substantial growth over the past two decades. This expansion has been driven by several key factors. Rising disposable incomes have led to greater healthcare spending, creating a substantial demand for medical devices. Simultaneously, government initiatives have fostered the development of domestic capabilities through investments in research and development, manufacturing, and infrastructure.
This supportive environment has enabled Chinese firms to produce a wider array of devices, catering to both domestic and international needs.
Current Market Position of Chinese Medical Device Firms in International Markets
Currently, Chinese medical device firms are actively seeking entry into international markets. Success in these ventures often hinges on factors like aligning with international quality standards, building strong brand recognition, and demonstrating consistent quality. While some Chinese firms have established a presence in international markets, challenges remain, particularly in areas like establishing trust and addressing concerns regarding regulatory compliance.
Key Players and Their Prominence in the Global Medical Device Landscape
Several prominent Chinese medical device companies have emerged as significant players in the global market. These companies, often backed by substantial government support and leveraging technological advancements, are expanding their reach beyond domestic borders. Their presence signifies a growing influence of Chinese companies in the global medical device landscape. Examples include companies focused on specific areas like cardiovascular devices, orthopedics, and imaging systems.
Technological Advancements and Innovations within the Chinese Medical Device Sector
Significant advancements are being witnessed in various segments of the Chinese medical device sector. This includes innovations in areas such as minimally invasive surgery, advanced imaging techniques, and sophisticated diagnostic tools. These technological advancements are often fueled by investments in research and development and the adoption of cutting-edge technologies. Furthermore, collaborations between Chinese firms and international partners frequently contribute to the rapid evolution of the industry.
Comparison of Regulatory Frameworks Between the EU and China Regarding Medical Devices
The regulatory frameworks governing medical devices differ significantly between the EU and China. The EU’s stringent regulations, often considered among the most rigorous globally, prioritize patient safety and efficacy. China’s regulatory landscape, while evolving, often presents a different approach, potentially prioritizing rapid market entry and domestic growth. These contrasting approaches underscore the importance of careful consideration of regulatory compliance when engaging in cross-border trade of medical devices.
EU Actions on Curbs
The European Union (EU) has increasingly scrutinized the participation of Chinese medical device firms in its public procurement processes. These actions, often framed as safeguards for public health and national security, reflect a complex interplay of economic, political, and regulatory considerations. The EU’s approach has been marked by a shift from a general framework to more targeted measures, and understanding the specifics of these actions requires careful examination of the motivations, impacts, and legal justifications.The EU’s actions on curbing Chinese medical device firms’ participation in public tenders are multifaceted and involve various regulations and procedures.
These measures aim to protect the EU’s healthcare system and ensure the safety and quality of medical devices procured for public use. Different criteria and standards may be applied to Chinese firms compared to those from other countries, potentially reflecting concerns about the origin and control of technology.
Specific Actions Taken by the EU
The EU has employed several strategies to curb Chinese medical device firms’ participation in public tenders. These actions encompass a range of regulatory adjustments and strategic decisions. Some actions are implemented on a case-by-case basis, while others are incorporated into broader EU regulations.
- Enhanced Scrutiny of Chinese Firms: The EU has introduced stricter evaluation criteria for bids from Chinese companies. These criteria often focus on the origin of components, manufacturing processes, and quality control systems, potentially reflecting concerns about compliance with EU standards and intellectual property rights. This approach requires rigorous due diligence and assessment of each bid.
- Specific Restrictions on Procurement: Certain EU member states have implemented specific regulations or restrictions on the procurement of medical devices from China. These measures may involve establishing minimum standards or disqualifying bids from particular Chinese companies based on specific criteria. This reflects a shift towards a more targeted approach, acknowledging the potential for variability in quality and standards among Chinese firms.
- Review and Re-evaluation of Existing Procurement Agreements: Existing procurement agreements with Chinese medical device companies have been re-evaluated to ensure compliance with EU regulations. This approach often involves audits and reviews of existing contracts, potentially leading to amendments or terminations of agreements that fail to meet the new standards. This is a proactive approach aimed at mitigating future risks.
- Collaboration with Other Countries: The EU has collaborated with other countries to develop and enforce common standards for medical devices. This collaborative effort seeks to establish a consistent approach to ensuring quality and safety in the global medical device market. This involves international agreements and shared best practices.
Justifications and Motivations Behind These Curbs
The EU’s justifications for curbing Chinese medical device firms’ participation in public tenders are rooted in concerns regarding public health, national security, and the integrity of the EU’s healthcare system. These actions are frequently presented as necessary safeguards against potential risks and vulnerabilities.
- Public Health Concerns: The EU aims to protect public health by ensuring the quality and safety of medical devices procured for public use. Concerns about the quality control standards and potential risks associated with devices from certain Chinese firms have been cited as justifications. This reflects a commitment to upholding the highest standards for public health.
- National Security Concerns: Some argue that the procurement of medical devices from specific Chinese companies poses national security risks. This concern often involves potential vulnerabilities in supply chains, dependence on foreign sources, and the possibility of technology transfer or intellectual property theft. This perspective suggests a need for strategic sourcing.
- Economic Considerations: The EU aims to foster a competitive environment for its own medical device industry. This perspective highlights the need to protect domestic firms and support their innovation and development, which might be threatened by the influx of cheaper medical devices from China.
Potential Economic Impacts
The EU’s actions could have significant economic impacts on both the EU and China. These impacts are complex and could manifest in various ways.
- EU Impact: The EU might experience disruptions in the supply chain for certain medical devices. However, this might also stimulate the growth of the EU’s own medical device industry, fostering innovation and job creation. The overall economic impact depends on the ability of EU companies to meet the increased demand and adapt to the changing market.
- Chinese Impact: Chinese medical device firms might face reduced market access to the EU. This could impact their revenue streams and future growth prospects. This may prompt a shift in strategies and an increased focus on other markets.
Legal Basis and Procedures
The EU’s actions are underpinned by EU law, particularly concerning public procurement, health regulations, and security considerations. The procedures for implementing these actions typically involve consultations, assessments, and legal reviews.
- Public Procurement Regulations: The EU’s public procurement regulations provide a framework for awarding public contracts. These regulations dictate the criteria for evaluating bids, ensuring transparency, and promoting competition. These regulations aim to balance national interests with the principles of fair competition.
- Health Regulations: EU health regulations Artikel the standards for medical devices. These standards ensure the safety and efficacy of devices intended for use on patients. These regulations aim to ensure the highest level of public health protection.
- Security Considerations: Recent EU regulations related to security risks in supply chains provide a legal basis for scrutinizing the origin and control of technology, ensuring the EU’s healthcare systems are not vulnerable to external threats. These regulations aim to balance national security with the needs of the economy.
Timeline of Actions
A detailed timeline of EU actions, including specific regulations, procurement decisions, and other relevant events, is crucial for understanding the evolution of these curbs.
- 2023-2024: Initial reports of increased scrutiny on Chinese firms and first instances of restrictions on specific procurement projects. This phase involved consultations and preliminary assessments.
- 2024-Present: Escalation of actions, including stricter evaluation criteria, specific restrictions on procurement, and the re-evaluation of existing agreements. This phase reflects a more proactive and targeted approach.
Potential Impacts and Consequences: Eu Backs Curbs Chinese Medical Device Firms Bidding Public Tenders
The EU’s recent actions to curb Chinese medical device firms’ participation in public tenders represent a significant shift in trade policy. These measures have the potential to reverberate through various sectors, impacting not only the companies directly involved but also the broader EU healthcare system, international trade relations, and global health standards. Understanding the multifaceted implications is crucial for anticipating both short-term and long-term consequences.
Economic Consequences for Chinese Medical Device Firms
These regulations can significantly impact Chinese medical device firms’ access to lucrative EU markets. Reduced participation in public tenders, a crucial avenue for market entry and establishing brand recognition, will likely hinder their growth and expansion within the EU. The loss of potential contracts may lead to decreased profitability and employment opportunities within these companies. Furthermore, the reputational damage resulting from perceived discriminatory treatment could negatively affect their global standing and future business prospects.
Examples include reduced investment in research and development (R&D), decreased export volume, and potential job losses within the Chinese sector.
Effects on the EU Healthcare Sector
The EU’s actions may lead to a reduced variety of medical device options available to European healthcare providers. A diminished pool of competitors might result in higher prices, limited product choices, and potential quality concerns if the competition is lessened. The EU might face difficulties in sourcing certain specialized or advanced medical devices, especially those not currently readily available from European manufacturers.
A potential shortage of certain critical medical devices could compromise patient care and potentially lead to increased costs. This also necessitates a review of the long-term sustainability of the EU healthcare system’s supply chain.
Ripple Effects on International Trade Relations
These actions could trigger retaliatory measures from China, potentially impacting other sectors of the EU economy. This escalation of trade tensions could create a broader negative impact on international relations, affecting cooperation on global health initiatives and hindering efforts to foster a more robust global health infrastructure. History offers numerous examples of trade disputes leading to wider economic repercussions, highlighting the need for careful consideration of such measures.
Implications for Global Health and Safety Standards, Eu backs curbs chinese medical device firms bidding public tenders
The EU’s approach could influence the direction of global health and safety standards. The potential for similar trade restrictions in other regions could set a precedent for more protectionist policies, potentially hindering the free flow of vital medical technologies and hindering efforts to improve healthcare access in developing nations. It also creates a critical question regarding the balance between national interests and global health initiatives.
The impact on global supply chains and innovation is a major concern.
Short-Term vs. Long-Term Impacts
The short-term effects may be immediately noticeable for Chinese firms in terms of lost opportunities and market share, while the EU healthcare sector might initially experience reduced competition and potential price fluctuations. Long-term consequences could include a decrease in innovation and potential quality concerns within the medical device industry. This shift in global trade dynamics could impact the accessibility of advanced medical technology, affecting both developed and developing nations.
The overall impact of these measures is difficult to predict accurately and depends on various factors.
International Relations and Trade Implications
The EU’s actions to curb Chinese medical device firms’ participation in public tenders represent a significant escalation in trade tensions. These measures carry far-reaching implications, potentially sparking retaliatory actions from China and disrupting global supply chains. Understanding the potential for trade disputes, alternative sourcing, and diplomatic considerations is crucial for navigating this complex situation.
Potential for Trade Disputes and Retaliatory Measures
China’s response to the EU’s actions could range from equivalent restrictions on EU medical device firms to broader trade sanctions. History provides numerous examples of retaliatory measures in trade disputes, highlighting the potential for a cycle of escalating restrictions. The severity of China’s response will likely depend on the perceived fairness and justification of the EU’s actions, as well as the broader geopolitical context.
China’s robust domestic medical device industry and its growing global influence suggest it has the capacity to retaliate effectively.
The EU’s move to restrict Chinese medical device firms from bidding on public tenders is a significant development, potentially impacting global supply chains. Meanwhile, as noted in the recent report “lutnick says us tariff levels china wont change” lutnick says us tariff levels china wont change , US trade policies seem set to remain firm. This suggests a growing trend of protectionist measures, potentially escalating tensions and further complicating international trade in the healthcare sector.
Potential Impacts on the Global Supply Chain
The EU’s actions, and any subsequent Chinese response, could disrupt the global supply chain for medical devices. A reduction in Chinese medical device exports to the EU could lead to shortages or price increases for certain products. This could impact hospitals, research institutions, and patients across Europe and globally. Furthermore, a reduction in Chinese imports could lead to a corresponding reduction in the global supply of components or raw materials used in medical device manufacturing, further affecting the availability of devices in global markets.
The EU’s move to restrict Chinese medical device firms from bidding on public tenders is a significant step, potentially safeguarding public health. However, it’s crucial to remember that factors like climate change are also profoundly impacting healthy pregnancies, as detailed in this article climate change impact healthy pregnancy. Ultimately, these measures aim to ensure high quality, safe products are available to citizens, while acknowledging the complex interplay of global health issues.
Potential Alternative Sourcing Strategies for the EU
The EU needs to diversify its sourcing of medical devices. This could involve strengthening existing relationships with other suppliers, particularly within the Asia-Pacific region, and potentially stimulating domestic production. Exploring opportunities in countries like India, South Korea, and Japan could provide alternative sources, though these options may not be a perfect substitute for the specific capabilities of Chinese manufacturers.
The EU must carefully consider the capabilities and reliability of these alternative sources and the potential for logistical and quality control challenges.
Diplomatic and Political Considerations
These actions involve significant diplomatic and political considerations. The EU and China must maintain open channels of communication to avoid further escalation of the trade dispute. The EU needs to consider the broader implications of these actions on its relationships with other countries, particularly within the context of global health cooperation and supply chain security. Potential trade disruptions could negatively affect the global fight against diseases and reduce access to life-saving medical technologies.
Potential Trade Friction Points and Potential Solutions
| Trade Friction Point | Potential Solution |
|---|---|
| Retaliatory tariffs and trade restrictions from China | Open communication channels and diplomatic engagement to seek mutually acceptable solutions. Exploring a multilateral approach with other countries to present a united front. |
| Disruption of global supply chains for medical devices | Diversification of sourcing strategies for the EU, strengthening partnerships with alternative suppliers, and promoting investment in domestic medical device production. Developing robust supply chain risk management strategies. |
| Geopolitical tensions and implications on global health cooperation | Promoting dialogue and understanding between the EU and China, focusing on shared interests in global health and sustainable development. Collaborating on issues like disease prevention and treatment. |
Public Health and Safety Considerations
The EU’s actions to curb Chinese medical device firms’ involvement in public tenders raise important questions about public health and safety. These measures aim to bolster the EU’s own medical device sector, ensuring a higher standard of quality and safety for its citizens. However, the implications for global supply chains and access to affordable medical technologies must also be carefully considered.These curbs are not a blanket rejection of Chinese products.
Instead, they represent a focused effort to prioritize EU manufacturers and ensure the highest quality standards for medical devices used in public health settings. The EU recognizes the crucial role of medical devices in public health outcomes and aims to improve these outcomes by strengthening its own regulatory framework.
Potential Impact on Public Health and Safety in the EU
These restrictions could temporarily impact the availability of certain medical devices, potentially leading to increased costs or delays in procurement. However, this is expected to be mitigated by the increased capacity of EU manufacturers to meet demand. In the long term, these measures are intended to improve public health by fostering a more robust and reliable EU medical device industry.
Increased competition and innovation among EU manufacturers could lead to more efficient and effective solutions for healthcare needs.
EU’s Approach to Maintaining and Improving Public Health Outcomes
The EU’s strategy centers on reinforcing its own medical device regulatory framework, thereby strengthening public health outcomes. This includes tightening quality control measures and enhancing transparency throughout the supply chain. By promoting a more robust domestic industry, the EU aims to achieve greater independence in critical medical technology procurement. This is particularly important for essential medical devices, ensuring consistent quality and availability.
Increased Safety and Quality Standards Within the EU Market
The EU’s actions aim to raise the bar for medical device quality and safety within the EU market. This is achieved by enforcing stricter compliance with EU regulations. A more rigorous approach to quality control and certification could lead to improved safety standards for patients using these devices. This proactive approach aims to prevent potential risks associated with substandard medical devices.
Mechanisms for Ensuring Quality Control and Standards for Medical Devices in Public Tenders
The EU likely employs several mechanisms to ensure quality control and adherence to standards in public tenders for medical devices. These include:
- Rigorous pre-qualification processes: Companies bidding on public tenders must demonstrate adherence to EU regulations and meet specific quality standards. This process likely involves audits, inspections, and the verification of manufacturing processes.
- Enhanced scrutiny of documentation: Thorough review of documentation from manufacturers, including quality assurance certificates and compliance records, is essential to ensure adherence to EU regulations.
- Independent audits and certifications: The involvement of independent bodies to audit manufacturers and certify devices is a crucial step in upholding standards and preventing fraud.
- Clearer labeling and traceability requirements: Detailed labeling and traceability systems enable clear identification of devices and facilitate rapid response in case of quality issues.
These measures are aimed at safeguarding the quality and safety of medical devices procured by public institutions.
Key Safety Concerns and EU’s Responses
| Key Safety Concerns | EU’s Responses |
|---|---|
| Potential for substandard medical devices to enter the EU market | Stricter pre-qualification requirements for suppliers, enhanced audits, and enforcement of EU regulations. |
| Increased procurement costs for medical devices | Strategies to foster competition among EU manufacturers, and potentially exploring alternative, compliant sources within the EU. |
| Disruptions in supply chains for specific medical devices | Diversification of supply sources and fostering domestic manufacturing capacity. |
| Potential for delays in medical device procurement | Streamlined procurement processes, and possibly, advanced planning for device needs. |
These responses are designed to mitigate the risks associated with the curbs while aiming for a higher standard of public health and safety.
Conclusive Thoughts
The EU’s decision to curb Chinese medical device firms’ participation in public tenders carries substantial implications for both the EU and China. Potential trade disputes, shifts in global supply chains, and adjustments in sourcing strategies are all likely outcomes. Ultimately, the long-term consequences for international relations, global health standards, and the future of the medical device industry remain to be seen.
This complex situation necessitates a nuanced understanding of the factors driving this action and its potential ramifications.